Abstract
Background
Patients (pts) receiving the BTK inhibitor ibrutinib (ibr) for CLL rarely achieve complete remission (CR) with undetectable minimal residual disease (U-MRD). Therefore, indefinite ibr maintenance therapy (Rx) is standard of care. Long-term Rx with ibr results in a cumulative risk of Rx discontinuation due to progression or toxicity. The risk of progression is highest in pts with complex karyotype and/or del(17p); some series suggest increased risk in pts with del(11q) or persistently elevated β 2-microglobulin.
The Bcl-2 inhibitor venetoclax (ven) is synergistic with ibr. We hypothesized that adding ven to ibr in pts at high-risk for CLL progression, who had been on ibr for more than 12 months (mo) would achieve U-MRD and allow Rx discontinuation.
Methods
We designed a phase II, investigator-initiated, response-adapted clinical trial with the addition of ven to ibr in pts (pts) with one or more high-risk features for disease progression who had received at least 1y of ibr regardless of line of Rx. Pts had detectable disease at time of study entry without meeting IWCLL criteria for progression. High-risk was defined as presence of at least one of: del(17p) or TP53 mutation; complex karyotype; del(11q); or elevated β 2-microglobulin. Ibr was continued at 140-420mg/d and standard initiation and escalation of ven was performed to the target dose of 400mg/d. Rx with combined ibr and ven continued for a maximum duration of 24, 28-day cycles (C). Pts had bone marrow (BM) evaluation for MRD by flow cytometry (sensitivity 10 -4) and CT scan for re-staging every 6mo. Pts in CR with U-MRD on two consecutive evaluations, 6mo apart, stopped ven, but could continue ibr at physician discretion. Pts who were MRD+ post-C24 continued ibr maintenance.
Results:
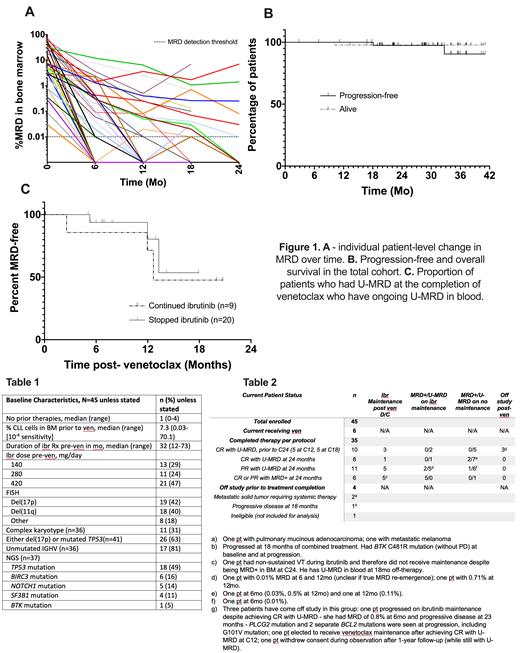
We report the results of the first 45 pts enrolled. All patients are now assessable for the primary endpoint, which was U-MRD in bone marrow post-C12. Pre-treatment patient characteristics are shown in Table 1. Of note, 22/45 patients were receiving ibrutinib as initial therapy. 63% of pts had del(17p) and/or mutated TP53.
On intent to treat analysis, 17/45 pts (38%) post-C6 and 26/45 (57%) post-C12 achieved U-MRD. Best cumulative rate of U-MRD in BM was 33/45 (73%), Figure 1A. Two pts (4%) were in CR prior to ven. Of the remaining pts, 23/43 (53%) pts improved their response to CR/CRi during ven treatment.
Median follow-up is 29 mo. Thirty-five patients completed treatment per protocol. Twenty-nine pts had U-MRD at the completion of planned study treatment. Of these 29 patients, 10 stopped ven prior to C24 per protocol (5 after C12 and 5 after C18) after achieving U-MRD CR on two consecutive response assessments. At completion of ven, 9/29 pts continued ibr at physician discretion (5/9 with del(17p)). Two patients have progressed (Figure 1B), one during combination therapy and one at 23mo post-ven during ibrutinib maintenance. During follow-up, blood MRD analysis was obtained q6 mo (Figure 1C); 24 pts have had at least one measurement, 6 of whom have had confirmed MRD re-emergence (one subsequently progressed). Information on the current patient status is detailed in the table.
Treatment was well-tolerated. The most common AE was diarrhea in 27 (61%) pts, grade 1-2 in all but 1 pt. The most common grade 3-4 AE was neutropenia, seen in 22% pts. No febrile neutropenia was seen. Three patients developed grade 3 infections (pneumonia, pyelonephritis, skin abscess). No patient stopped treatment due to toxicity.
Conclusions:
Consolidation ven added to ibr in pts with high-risk CLL was well-tolerated and achieved cumulative BM U-MRD4 rate of 73%. This allowed Rx discontinuation in a significant proportion of patients. Risk of disease progression during combined treatment was very low. Off-treatment follow-up continues to mature; at a median of 12 mo post-ven follow up, most pts who attained BM U-MRD have ongoing U-MRD in blood. A second cohort restricted to 45 high-risk pts with TP53 abnormalities or complex karyotype is accruing.
Thompson: Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Amgen: Other: Institution: Honoraria, Research Grant/Funding; Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Gilead: Other: Institution: Advisory/Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding. Ferrajoli: Janssen: Other: Advisory Board ; AstraZeneca: Other: Advisory Board, Research Funding; BeiGene: Other: Advisory Board, Research Funding. Jain: Incyte: Research Funding; Genentech: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; TG Therapeutics: Honoraria; Adaptive Biotechnologies: Honoraria, Research Funding; Pfizer: Research Funding; Janssen: Honoraria; ADC Therapeutics: Honoraria, Research Funding; Pharmacyclics: Research Funding. Kadia: Cure: Speakers Bureau; Sanofi-Aventis: Consultancy; Novartis: Consultancy; AstraZeneca: Other; Jazz: Consultancy; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Liberum: Consultancy; Pulmotech: Other; Pfizer: Consultancy, Other; Cellonkos: Other; Ascentage: Other; Genfleet: Other; Astellas: Other; BMS: Other: Grant/research support; Amgen: Other: Grant/research support. Bose: Blueprint Medicines: Honoraria, Research Funding; Kartos Therapeutics: Honoraria, Research Funding; Novartis: Honoraria; CTI BioPharma: Honoraria, Research Funding; Sierra Oncology: Honoraria; Celgene Corporation: Honoraria, Research Funding; Astellas: Research Funding; BMS: Honoraria, Research Funding; Incyte Corporation: Honoraria, Research Funding; Pfizer: Research Funding; Constellation Pharmaceuticals: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding. Pemmaraju: Sager Strong Foundation: Other; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Blueprint Medicines: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Other, Research Funding; DAVA Oncology: Consultancy; CareDx, Inc.: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Plexxicon: Other, Research Funding; Samus: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Clearview Healthcare Partners: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; LFB Biotechnologies: Consultancy; Bristol-Myers Squibb Co.: Consultancy; Incyte: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Springer Science + Business Media: Other; Roche Diagnostics: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; MustangBio: Consultancy, Other; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Short: NGMBio: Consultancy; Novartis: Honoraria; Amgen: Consultancy, Honoraria; Takeda Oncology: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Astellas: Research Funding; AstraZeneca: Consultancy. Wierda: Miragen: Research Funding; Karyopharm: Research Funding; AstraZeneca: Research Funding; Sunesis: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; GSK/Novartis: Research Funding; Genentech: Research Funding; Juno Therapeutics: Research Funding; Gilead Sciences: Research Funding; KITE Pharma: Research Funding; Acerta Pharma Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Xencor: Research Funding; Janssen: Research Funding; Cyclacel: Research Funding; Loxo Oncology, Inc.: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal